Stories in Science Special Series
A Lab-Coat AND Hiking Shoes
I’ve spent my professional life juggling two careers. First, as a lab neurobiologist, I study rats and cultured neurons, trying to understand how stress damages the brain. Second, as a field primatologist, I study the effects of social stress and social subordination on health in wild baboons, living in a national park in East Africa. I’ve spent more than thirty years doing the latter gig during the summers, doing the lab neuroscience the rest of the time, and it’s been a blast.

– Robert Sapolsky, PhD –
John A. and Cynthia Fry Gunn Professor and Professor of Neurology and Neurosurgery at Stanford University
I have spent my professional life juggling two careers. First, as a lab neurobiologist, I study rats and cultured neurons, trying to understand how stress damages the brain. Second, as a field primatologist, I study the effects of social stress and social subordination on health in wild baboons, living in a national park in East Africa. I’ve spent more than thirty years doing the latter gig during the summers, doing the lab neuroscience the rest of the time, and it’s been a blast.

Robert Sapolsky
The desire to be a primatologist came first. I was about eight years old when I settled on that. Well, more precisely, I decided I wanted to be a mountain gorilla. They live extremely peaceful, family centered lives in these beautiful, high altitude equatorial mountains – who wouldn’t want to be one of them? I eventually came to terms with the fact that this wasn’t going to happen, and settled on the idea of going to study them. In my years as a primatologist, I’ve noticed there’s two general patterns to people who do fieldwork. The first type grew up out in some exotic locale – their parents were field researchers, or missionaries, adventurers, some such thing, and it was natural that they head in their direction. The second grew up in some godawful urban setting, where all they wanted to do was get the hell out of there as soon as possible. I was in that second category, growing up in one of the less delightful neighborhoods of Brooklyn, and spending all my time in the natural history museum, thinking about field work.
I was all set on this path. By high school, I had already read tons of primatology, was sending embarrassing fan letters to primatologists all over the world, and was teaching myself Swahili in preparation. Two big shifts in my plans came when I got to college.
I had gone to Harvard to study with this guy who was one of the grand pooh-bahs of field primatology. My first semester, freshman year, I was all set to sit at his feet in a bunch of classes. And from out of the blue, he had a mild heart attack and cancelled all his classes (he recovered and lived another thirty years). Stranded, somewhat on a whim, I filled in one of those slots with an intro neurobiology class. I was blown away. My orientation had been to think about the evolution of behavior and of ecological influences on behavior, and suddenly I was intensely taken with thinking about the neurobiological and endocrine bases of behavior. Which is mighty hard to study out in the field. Was I going to be a field primatologist or a lab neurobiologist? Was I going to spend the rest of my life wearing a lab coat or hiking shoes?
The incredibly lucky outcome is that I’ve gotten to regularly do both. I worked out some really crude techniques for studying neuroendocrine function in wild primates, while doing more traditional lab neurobiology concerning behavior. Each has fed somewhat on each other. When observing something in field and thinking, I wonder if Brain Region X has something to do with this?, I could then study that in the lab. When observing something in the lab and thinking, I wonder if things work this way in animals living in their natural habitat?, I could study it in the field.
This necessitated my second shift, which was that I was not going to be able to go anesthetizing wild gorillas with blowgun darts in a rainforest in Rwanda. I needed a primate that lived on the ground, in open savanna, was not endangered and, frankly, had big enough of rear ends so that I’d have a fighting chance of a successful shot with my blowgun. Baboons made lots of sense. I’d come to terms with not becoming a mountain gorilla, and now I came to terms with not studying mountain gorillas and focusing on baboons in the Serengeti instead. I went off to a year of fieldwork a week after college graduation, found an advisor in grad school who, despite being a neurobiologist, was okay with me disappearing from the lab 3 – 4 months a year to do my fieldwork, and had been oscillating back and forth ever since. I get to wear both a lab coat and hiking shoes; I’ve been extremely fortunate; this is a wonderful way to spend your life.
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
Civic Science Observer1 week ago
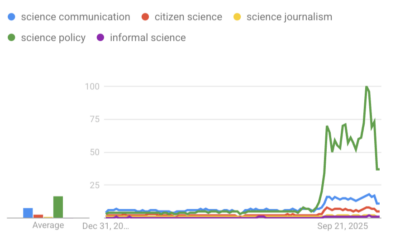
‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress