Stories in Science Special Series
Two Steps Forward, One Step Back

– Edritz Javelosa –
[dropcap]T[/dropcap]he luscious vegetation, crawling critters, warm beaches, and the tropical climate of the Philippines – an archipelago with more than 7,000 islands, tons of natural resources, and a wide range of biodiversity – was the setting to my childhood upbringing. Growing up in an area surrounded by nature was one of the many reasons that ignited my interest in science. My adventurous spirit to discover and learn continued to grow from childhood to adulthood, and who would have thought I would become a scientist.
I was 14 years old when I arrived in the United States. Despite migrating to the U.S., there was still a big push for me to become a nurse because this is a common career choice for many Filipinas. However, I decided to go against the trend and immersed myself into the realm of science. Coming from a low-income background, my choice to attend a major university created concerns for me and my family. Nonetheless, I enrolled at the University of Arizona and majored in Molecular and Cell Biology.
I became the first in my family to earn a college degree and later to attend graduate school. As a first-generation student, I have set myself up for high expectations

Edritz Javelosa
coming from my family and myself. This has created an immense amount of pressure. But there is also a lot of pride because I have achieved this important milestone in my life.
While my success has changed my parents’ view about my career choice, my path is uncommon among Filipinas. This is partly due to the lack of role models. In all my time at two large universities (University of Arizona and Stanford), I have not met a single Filipina faculty member in a STEM field.
My goals in life are constantly evolving, which is terrifying, but also simultaneously exciting.
At the University of Arizona, I came across the Ronald E. McNair Program for underrepresented students. Applying to and becoming a McNair scholar was a pivotal point for me. Through the McNair program, I found a research position in the lab of Professor Tsu-Shuen Tsao where I worked on the effects of AMPK (AMP-activated protein kinase) α on the stability of erythropoietin (a substance that promotes the production of red blood cells). This was truly my first real immersion to research in biology and a confirmation that being a scientist was what I wanted to pursue.
The following year, the McNair program funded my 2011 summer internship at Stanford University where I worked with Professors Craig Garner and Richard Reimer characterizing novel lysosomal markers. Why? We wanted to use the markers to map out the movement of the lysosomes in neurons.
My summer experience at Stanford gave me the confidence to succeed as a graduate student in a program of the highest caliber, which was key to my decision to apply for the Neuroscience PhD Program at Stanford. Currently, I am working in Dr. Richard Reimer’s lab exploring the effects zinc interaction with a protein and how this contributes to Parkinson disease.
When I am not doing bench work, I participate in activities advocating STEM to first-generation and/or low-income students. From my own experience being in this field for ten years, there is a need for an increase in the diversity and inclusion in academia and industry.
As a Filipino woman in a STEM PhD program, I have yet to meet an ethnic and gender matched role model, which is partly the reason that has instilled in me a strong commitment to enhancing diversity in STEM to benefit others who have backgrounds similar to mine through teaching and mentoring. My years at Stanford have given me opportunities to be involved in programs where I can give guidance, support, and be a role model to the next generation of bright scholars from non-traditional backgrounds.
In this world I learn about cool, different, odd, and new things everyday either in science, education, technology, art, etc. The curiosity that I had as a child never left. Instead it only grew and made me eager to see, taste, smell, hear, touch, and learn from all of those experiences. My goals in life are constantly evolving, which is terrifying, but also simultaneously exciting.
As I finish my last year at Stanford, I plan to take courses that are outside of my field and continue my involvement in education outreach. I eagerly anticipate building more knowledge of the world and to life fulfilling adventures as I continue to develop to grow as an individual, a scientist, and a mentor. I encourage everyone to discover what s/he values, be curious and ask questions, and create your own adventure.
Edritz Javelosa is a graduate student in the Neuroscience Program at Stanford University. Cover image is by Sonorax from Pixabay | CC0 Creative Commons
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
 Civic Science Observer1 week ago
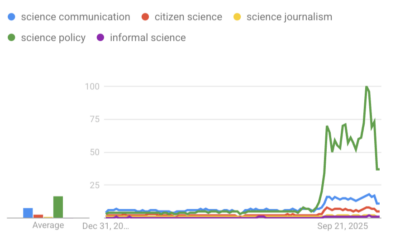
Civic Science Observer1 week ago‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress