Stories in Science Special Series
My Journey with Science

Anonymous
I have been interested in science from a young age when I liked to build intricate contraptions that I called “inventions”. These gadgets made from rubber bands and other household items helped me explore how moving parts worked and the basics of physical science. I remember when I discovered that I could reflect the images from my NES Duck Hunt game across mirrors in my basement and still hit the target. I would set up several mirrors in series reflecting the game on the television to test the limits of light reflection across extended distances. And when I got older, my parents bought science kits and taught me concepts such as electromagnetism by winding a copper wire around a nail, connected to a power source. I became fascinated with the hidden secrets behind everyday products and machines that we surround ourselves with.
By the time I reached college, I knew that I wanted to use my talent for discovery and design to help people. So I began studying for a career in biomedical engineering. I figured this track would allow me to figure out new ways to advance the medical field by designing and building cutting edge products and technologies. I really enjoyed the exhausting two-semester senior design projects that we developed in our biomedical engineering department. It was exciting to see a project go from idea to product. The concept of taking the initial concept to design development and finally building the contraptions we envisioned was amazing. For example, our team built a remotely operated monitor-lift created for a biomedical laboratory that used eye-tracking algorithms to assess diseases, as well as a custom-designed device to assist a patient with limited dexterity in opening oil-paints that the patient used in his career as an artist. It was highly rewarding to see our products in use to solve the specific problem they were designed for. My interest in these courses encouraged me to stay on for a master’s degree in biomedical engineering where I was able to learn even more about biomedical design and discovery.
By the time I reached college, I knew that I wanted to use my talent for discovery and design to help people.
One challenge I have had in my scientific tract is being interested in too many things at once and not focusing on a specific career goal. I think that the biomedical engineering track can sometimes add to this problem since you learn a little bit about almost everything but don’t focus in depth on one scientific niche as you would in another form of engineering or science. This is okay for many students who knew exactly what they wanted to do after they graduated. But for me, I just figured I would explore all of my options in the scientific realm when the time came for applying for jobs. This was probably the biggest mistake I made and my warning to biomedical engineering students: make sure you have an internship during college that will likely place you into your first engineering position. If you graduate and hope to find a position at a company, you might be presented with an interesting catch-22. When you search for an entry level position, the majority of postings require several years of experience. So, the best way to make sure to get your foot in the door is to have an internship during your junior/senior college years that can have the opportunity of becoming your first post-graduation job. My college internship was in an academic lab, which is not as useful if you’re trying to get a position at a biomedical corporation.
Since college, I have worked as a research scientist in academia. It has been very rewarding. I have had the opportunity to work with cutting edge technologies at Harvard, MIT and the University of Minnesota in electron microscopy, optogenetics, and non-invasive neuro-modulation. I have learned that academia fits my creative personality quite well; if you have an idea you can go ahead and do it that same day and discover what happens. In industry, you rarely get to see a project go from design to product, and you will most likely only have your hands in one small component of a project. In academia, you get to think up solutions to problems, build and test them, and see those ideas to fulfillment. In my experience, I’ve also learned that there is little place for a master’s degree in academia. If one plans to advance an ambitious research career at a university, it may be more useful to first attain a PhD. Luckily, our current project already has a significant collaboration with a local biomedical company. If the science continues to go well through this collaboration, there may be opportunities to join the biomedical company or even build our own start up. For now, I am happy dabbling in the latest biomedical technologies and will see if my career eventually bridges over to industry or stays within academia.
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
 Civic Science Observer1 week ago
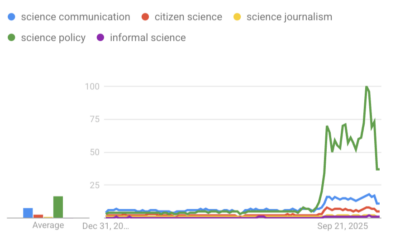
Civic Science Observer1 week ago‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress