Stories in Science Special Series
Forever a scientist: How I found my career niche

– Julia Bates –
“No Julia, you are still a scientist. You will always be a scientist”.
[dropcap]T[/dropcap]hose were the words spoken by my mentor Professor Jenny Martin, as we sat drinking coffee in a sunny café in my hometown of Brisbane, Australia. I was trying to explain to her my thoughts and feelings on leaving the bench to pursue a career in science writing.
But if I’m not scientist, who am I? I still pondered.
But let’s go back to where my science career all began—high school.
Forever the science student
Ah, welcome to high school and the immense pressure of deciding what you want to be when you “grow up”. Seriously, who can know at such a young age? I sure didn’t.
However, in my final couple of years, I had worked out which subjects I liked best—I chose three science subjects (physics, chemistry, and biology) and two maths subjects. My friends thought I was crazy (and a bit boring), but I loved that there was always the “right answer” in science. It’s funny how I thought that then, having learnt we are constantly finding new “right answers” in science.
Because I loved biology and chemistry, and was doing quite well academically, I was advised that being a medical doctor might be a good fit for me. The only problem—I easily fainted even at the thought of blood. My 5-day work experience stint at the local hospital confirmed my suspicions when—to my complete embarrassment—I passed out in the pathology lab. And don’t even get me started on the operating theatre!

Julia Bates
So, where did that leave me? Still loving everything to do with science, I enrolled in an undergraduate science degree. In my third year, I sat down to a lecture given by Professor Jenny Martin on Protein Crystallography and Drug Design. She was confident, humorous, intelligent, and her research sounded like a cool mix of computational work and working at the lab bench. It was then I had my “aha” moment. A research career! I could combine my desire to “help people” medically with my love of learning (and avoid the blood).
My science segue
About ten years later, and now living in New Zealand, things started to shift. I felt unsettled. My research career was going pretty well if you looked at it on paper. I had the following:
- An undergraduate degree in biomedical science
- 3-years experience at a pharmaceutical company
- A PhD in structural immunology
- More than 10 articles published in scientific journals
- Received the Victorian Premier’s Award for Medical Research
- Obtained a postdoctoral fellowship to fund my salary for the next four years.
But I wasn’t happy. I didn’t have the passion and drive for lab work that my colleagues seemed to have. I was in my 30s, single, and constantly questioning myself.
Was I really smart enough? Was this really what I wanted to keep doing for the rest of my life? How could I keep moving from country to country on short-term contracts, AND settle down to have a family? Was I better suited to another career?
I enjoyed reading and writing about science, but failed experiment after failed experiment was getting me down. I was taking it too personally. I had forgotten the very definition of the word “re-search”—to search again, and again (and again).
In the words of Roxette, “listen to your heart”
So my heart was saying I needed to change. But to what? Like most things, I decided to tackle the question pragmatically. First, an online career quiz, followed by an appointment with the career counsellor at the university. The answer? “Accountant”… or a close second: “scientist”. I laughed. Neither of these options excited me.
Back to the drawing board.
“What about patent law?” my sister (a lawyer) suggested. “You can still read about all the cool science discoveries?”
She gave me one of her textbooks—but after many failed attempts at reading about intellectual property (and getting distracted by Facebook instead), I realized my heart wasn’t in it.
Upon voicing my frustrations to a colleague in New Zealand, she suggested medical writing. She knew someone who had felt the same way as me, and who now works from home as a medical writer.
What is this medical writing, I thought to myself and started googling. It sounded perfect: I would be able to use my science background, but step out of the lab and have some control.
I now had a direction, and excitedly started applying for jobs. My heart was screaming yes!
All you need is a little bit of luck
I had decided upon my career aspiration, but unfortunately getting there wasn’t that straightforward. I applied for numerous science and medical writing positions, without success.
“You have no experience,” they said.
Feeling dejected, I moved back to Australia to another job in a lab. I completed a Graduate Certificate in Editing and Publishing at nights and on the weekends, with the hope a qualification would give me an edge. But still, no luck.
Through a chance meeting with the communications manager, a stint volunteering, being persistent (probably to the point of being a bit of a nuisance), and then some good fortune, I finally got a job in my university’s communications department. Here, with support and training from my new boss, I tried my hand at all types of writing: from media releases to annual reports. I was still engaged with science, but finally felt I was doing something I was good at.
Almost a year later, with my partner travelling overseas all the time for work, I decided to make the leap into the freelance world (although, my supportive hubby was providing me with a sturdy safety net).
My fancy-free freelance life
I now happily work as a freelance science and medical writer. I get to read about so many different areas of science and medicine. I get to travel with my hubby, working from the hotel bedroom when we are away. I am my own boss and I am never bored. Each day, I am working on something different (you can read more about a day in my life here).
As my hubby says, “so basically, you do assignments everyday?”
Yes, yes I do, and I love it. I will forever be a student who loves learning about the world around me. And Jenny is right; I will forever be a scientist—just one who has found a slightly different niche.
About the author: Julia Bates, PhD, has over 15 years experience in the research sector. Although she originally trained as a structural biologist, she now runs her own science writing and editing business, Science Write (www.sciencewrite.com.au).
Cover image is by Arek Socha from Pixabay | CC0 Creative Commons
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
 Audio Studio1 month ago
Audio Studio1 month ago“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
Civic Science Observer1 week ago
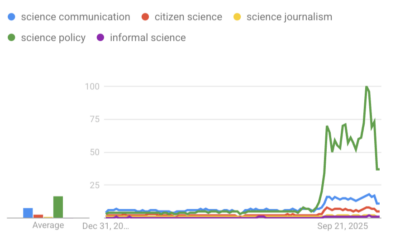
‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress