Stories in Science Special Series
Crystals are a Girl’s Best Friend

– Julia Bates –
[su_boxbox title=”About” box_color=”#262733″]Julia Bates, PhD, has over 15 years experience in the research sector. Although she originally trained as a structural biologist, she now runs her own science writing and editing business (www.sciencewrite.com.au) from her home in Brisbane, Australia.[/su_boxbox]
“Oh look at my pretty crystals!” I squealed in delight.
“Remember, the pretty ones aren’t always the best,” the postdoc sitting next to me commented.
[dropcap]I[/dropcap] rolled my eyes, even though I know he was right. Looks aren’t everything; it was what was on the inside that counted. I looked down the microscope again at the perfectly formed, glistening, diamond-shaped crystals. Please be good, I pleaded with the protein gods.
It had taken me over two years to get to this point. But I am still hopeful. Hopeful that these crystals will be the ones: the ones to help me “see” how the tiny molecules within our immune system work to eliminate viruses or, unfortunately, cause organ transplant rejection.

Julia Bates
Proteins are too tiny to see with our naked eye, or even under a traditional microscope, so we have to use another method: X-ray crystallography. I know it sounds like some sort of fortune telling, crystal ball, hocus pocus, cr*p. But this is serious stuff. This was how Rosalind Franklin figured out what DNA looks like in the early 1950s.
The technique is now used by many scientists around the world to understand the structure and function of proteins and small molecules, which are at the very heart of life. Professor Elspeth Garman, a structural biologist, does a fantastic job at explaining X-ray crystallography in this short video.
With the help of constantly evolving technology, we have been able to visualize the structures of many proteins from all walks of life. The majority of these structures are freely available for download from the Protein Data Bank. Yet, we still have a long way to go—the human body alone is thought to contain over 50,000 proteins.
So I stared at my crystals, thinking back on the journey to get here. I had overcome a number of battles: I had fought hard to produce large enough quantities of the protein in the lab to be able to conduct these experiments; I had made sure my sample was extremely pure; I had proven the proteins were still functional, even though they were no longer within their natural cellular environment; and finally, I had found a way to turn them from a solution into a crystalline form.
Why a crystal? I guess the easiest way to think about it is that the crystal acts as an amplifier. Instead of trying to get information from a single protein molecule floating around in solution, a crystal will contain millions of copies of the same protein, ordered in a specific way, giving us a much stronger and clearer picture. When we fire X-rays at the crystal, they will be scattered by the atoms of the protein in a particular way, providing us with a “diffraction pattern”. This pattern gives us information about the 3D structure of the protein molecule.
I had tested thousands of conditions, trying to coax my protein to form a crystal. I had varied the pH, added different salts, and used a myriad of precipitants, before landing on the perfect mix to grow the diamond-shaped crystals. But were my proteins ordered well enough within the crystal to create a good diffraction pattern?
I walked next door to the room containing the X-ray machine. Damn, someone is using it, I thought. I checked our online booking system—I would have to wait two more days before the machine was free. Feeling impatient, I set up some more experiments, aiming to grow more of the same crystals for when the time came. I went home. I tried to distract myself with wine and binge watching Sex and the City.
Two days later, I came into the lab early and sat back down at the microscope in our temperature controlled “crystal growing” room. I only had about seven “good-looking” crystals, ranging from about 0.1 to 0.5 mm in size. I was nervous. I took some photos as evidence in case something went horribly wrong. I started to doubt myself. I looked at my hands—they were fully shaking, not ideal for manipulating the delicate crystals.
I went back out into the main lab. “I’m too stressed to do it,” I confessed. “Can someone please help?”
An experienced postdoc laughed, and then kindly said they would help. If you have too much invested, you are more likely to make a mistake, they agreed. I stood nervously to the side, as they carefully and confidently fished out the tiny crystals, before mounting them in the X-ray machine.
We both looked at the camera used to align the X-rays, and breathed a sigh of relief. The crystal sat proudly in its position: its diamond shape maintained, it’s glossy surface immaculate, with not a crack to be found.
“Thank you”, I said gratefully to my co-worker.
But now came the true test. The moment, we had all been waiting for. We fired some X-rays at the center of the crystal. I fidgeted. My heart was pounding. But then, there it was: a stunning diffraction pattern. Today, the protein gods were on my side.
Within a couple of weeks, I sent some crystals to a more powerful light source (a synchrotron) to collect even better data. Then, using powerful computational software, we were able to “see” how the proteins were interacting and how every tiny atom was positioned within the crystal for the very first time. You can learn more about how the diffraction pattern gives us information about the structure of proteins here.
Being one of the first people in the world to “see” how these proteins interact with each other at the molecular level is an indescribable feeling. I was so proud to be part of a talented research team that led to this discovery, and proud to tell the world what we had found using my “pretty” crystals.
We have all seen beautiful crystals in our life, including stunning diamonds made of pure carbon. While my protein crystals were smaller in size than the rock I now wear on my left finger, the crystals are much more valuable to me. These sparkly crystals can give us a glimpse at the tiny building blocks of life. They tell us how enzymes work, how blood transports oxygen, and how to design new therapies to treat disease.
To me, these types of crystals truly are a girl’s best friend.
Cover image is by Thomas B. from Pixabay | CC0 Creative Commons
Metrics
Sessions
Total number of Sessions. A session is the period time a user is actively engaged with the page.
Visitors
Users that have had at least one session within the selected date range. Includes both new and returning users.
Page views
Pageviews is the total number of time the article was viewed. Repeated views are counted.
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
 Civic Science Observer1 week ago
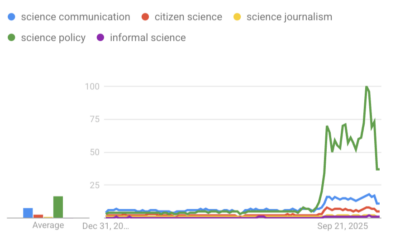
Civic Science Observer1 week ago‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress