Stories in Science Special Series
Alice Augusta Ball: Chemical Drug Pioneer
Historians of African-Americans in science tend to focus on figures like Benjamin Banneker and George Washington Carver. But there are so many more.

Sibrina Nichelle Collins
[su_boxbox title=”About”]Sibrina Collins is an organometallic chemist and former writer and editor for the American Association for the Advancement of Science in Washington, D.C. In July, 2016, she became the first executive director of the Marburger STEM Center at Lawrence Technological University in Southfield, Michigan. This article was originally published on Undark on May 12, 2016 by Sibrina Nichelle Collins. Read the original article.[/su_boxbox]
When it comes to African-American scientists, historians tend to focus on figures like Benjamin Banneker and George Washington Carver. Banneker, an almanac author and Carver, an agricultural chemist, of course did great work, but the narrative shouldn’t stop with them.
African-Americans have been making important contributions to the science and technology fields for centuries, but their stories are often overshadowed or forgotten. Thomas Edison is said to have invented the light bulb, but it was Lewis Latimer who improved upon his original design. Dr. Henry Aaron Hill and Dr. Percy Julian started their own chemical companies in the 1950s and 1960s, creating job opportunities for women and people of color.
These scientists are not important because of their race, but that is a reason they’ve historically been overlooked. This editorial series will serve as a platform to increase recognition and explore the remarkable intellectual contributions of these “unsung” African-American scientists. As the reality of being eclipsed is even more pronounced for women, I begin by telling the story of chemical drug pioneer Alice Augusta Ball.
Alice Augusta Ball was born in Seattle in July of 1892. Her grandfather James was a famous photographer and her father was a lawyer. She had two older brothers, Robert and William, and a younger sister, Addie. After spending two years in Hawaii, she and her family moved back to Seattle following her grandfather’s death in 1905.
After graduating from Seattle High School in 1910, Ball earned her bachelor’s degree in pharmaceutical chemistry from the University of Washington. She went on to earn a second degree in pharmacy two years later. With her advisor Professor William M. Dehn, she co-authored a paper published in the Journal of the American Chemical Society, a highly unusual accomplishment for African-American women at the time.
From there, Ball returned to Hawaii to earn her master’s degree from the College of Hawaii (now known as the University of Hawaii), in 1915. Subsequently, she was hired as a chemistry instructor, becoming the first African-American and the first woman to hold this position at the school. During this time, she began to conduct research into developing a treatment for Hansen’s disease, or leprosy, an infectious disease that affects the skin, nerves and mucous membranes.
When Ball began her research, chaulmoogra oil had been used to treat the disease outside of western medicine for hundreds of years. It proved to be moderately effective as a topical agent, but injection would be better. At the request of Dr. Harry T. Hollmann, assistant surgeon at Kalihi Hospital, Ball successfully isolated the ethyl esters from the oil to make an injectable form to treat the disease. Her research efforts helped treat countless leprosy patients up until the 1940s when sulfone drugs were introduced. Sadly, Ball died in 1916 at the age of 24.
After her death, the president of the College of Hawaii, Dr. Arthur Dean, continued her research without giving her credit for the discovery. Hollmann later published a paper in 1922, giving Ball the proper credit she deserved.
Today, multiple-drug regimens are fairly effective at treating leprosy and, if the disease is caught early enough, long-term disability can be avoided. Although leprosy has not been fully eliminated — the WHO reported 214,000 new cases in 2014 — a target of less than 1 case per 10,000 people was reached in 2000.
Dr. Richard W. Truman, a microbiologist with the National Hansen’s Disease Program at Louisiana State University, indicates that genomics has played a significant role in treating the disease, specifically being “useful for detection of infections and sequencing technology.”
Recognizing the influence of Ball’s work, Paul Wermager, retired Science/Technology Reference Department Head at the University of Hawaii at Manoa Library, hopes to write a biography on her in the near future. “Not only did she overcome the racial and gender barriers of her time to become one of the very few African-American women to earn a master’s degree in chemistry, [but she] also developed the first useful treatment for Hansen’s disease,” said Wermager. “Her amazing life was cut too short at the age of 24. Who knows what other marvelous work she could have accomplished had she lived.”
In 2000, the University of Hawaii honored Ball with a plaque mounted on the only chaulmoogra tree on campus. In 2007, they posthumously awarded her with the Regents’ Medal of Distinction.
This article and cover image was originally published on Undark. Read the original article.
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
Civic Science Observer1 week ago
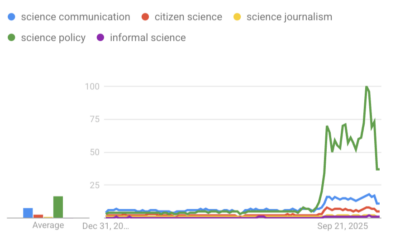
‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress