Stories in Science Special Series
Breaking the surface: Lessons on resilience and rebuilding from planarians
Divya Shiroor is a vet grad student at Cornell University. She is a foodie, dancer, bookworm, blogger, dog mom, and twin! Her recent first-author publication titled “Injury Delays Stem Cell Apoptosis after Radiation in Planarians” was published by Current Biology on May 7, 2020. Her narrative below explores the unwritten stories behind the publication. You can follow Divya on Twitter @DivyaShiroor.
Are you familiar with the iceberg illusion? that visible part of the iceberg peeking magnificently above the ocean? That’s success. Silently lurking beneath is a giant hunk of ice – a combined mass of failure, disappointment, exhaustion and pain, invisible to all but the iceberg. With my first-author research publication hot off the press, my tiny wedge of ice is now entering the sunlight. This narrative however is about that unseen, unspoken mass underneath, that each of us believes we singularly carry around.
I have the privilege of studying incredible flatworms called planarians. I’m enchanted by these worms for the same reason every other person that knows about their superpower is-their crazy ability to regenerate. My first encounter with planarians was in the early days of graduate school, when my advisor, a newly minted PI, showed us how these worms use special cells called stem cells to recover and regenerate from virtually any injury. When the first image of this squint worm flashed across the screen, I was in love. My stars must have aligned just right, and after a two-month rotation, I started out as the first official graduate student of our lab.
When I look at my publication today, I can’t help but be struck by how 5 years of blood, sweat and tears coalesce so tidily into 5 pages of a journal. I wonder how differently things might have turned out, had I not had two amazing lab mentors pushing me along.
I kicked off my research like most others, by reading pertinent literature to come up to speed on planarian biology. It was while I was on this quest that I came across a curious result, tucked away in the supplementary data of a seminal paper. I have just waxed eloquent about how these worms regenerate because their stem cells respond rapidly to injury. This piece of data showed however that in a particular scenario, these incredible stem cells completely failed to react like they should. I didn’t know it then, but latching on to this curious observation would result in the birth of a project that gave me my first paper, and will one day give me a Ph.D.
I realize I make it sound like I had an instant eureka moment, but the actuality of it was far less dramatic. While I couldn’t get that result out of my head, I couldn’t really define why it stuck with me either. I had an extensive list of reasons explaining why it might not be worth the attention I was giving it. I didn’t know the literature well enough; I was too green behind the ears; it was supplemental data so it probably wasn’t important anyway; if there was something to it, someone else would have spotted it by now; A lot has been said about feeling like an imposter in science and I don’t have much to add, except to say that it almost kept me from pursuing my dream project. That voice telling me it was impossible that I spotted something interesting was loud, and I would have yielded had it not been for my advisor. We discussed the result and she told me to explore it, a brave move for a 2-month-old PI with an already well-developed project ready to go.
Once I began to accrue data, I realized that we had stumbled upon an exciting stem cell response in planarians – their ability to withhold from certain death.
Adding to my confused feelings of inadequacy was my inexperience at the bench – something I carried with me heavily. I didn’t fully understand the science behind the techniques I needed for my experiments. My minimal molecular/genetic training before starting graduate school meant that I wasn’t always equipped with the language needed to make sense of the science. When my advisor cottoned on to just how much I was struggling, she jumped on board to help out. We came up with a system where I would select one technique that we used in the lab and spend hours on google teaching myself everything about it. I would then relay to my advisor all that I had learned, and we’d discuss things that still didn’t make sense to me. While I felt incredibly incompetent at the time, learning how to figure things out turned out to be a great anti-dote that fueled early progress with my project.
Once I began to accrue data, I realized that we had stumbled upon an exciting stem cell response in planarians – their ability to withhold from certain death. In order to definitively examine this however, we needed to quantify dying stem cells in these animals; this required us to either develop a new technique, or hone an existing one. This was my first real tryst with how slow, frustrating and defeatist science can be. I knew I’d never be able to publish my story without figuring this out, and there were long stretches where I was convinced we’d never get there. I finally managed to get one protocol to work, only to realize that it wasn’t sensitive enough to detect what we needed to determine. It took over two years of failing to finally nail down a technique that allowed us to efficiently quantify stem cell death in planarians – a crucial step for our publication. If I didn’t have an amazing advisor and a brilliant post-doc cheering me on, I’m not sure I’d have crawled through those years. Science is ALWAYS a team effort, and I will never take for granted how much of its outcome depends on the people around you.
Once we had identified a technique to measure stem cell death, we found that in certain scenarios – like when faced with an injury – planarian stem cells destined to die will pause this process, and choose to persist instead. We were excited about our discovery and our next hurdle was putting this story out into the world. So much of the fate of a lab hinges on its publications, and as a new lab we were still waiting on ours. This gave the final task of putting a paper together a constant, exhausting sense of urgency. After an unhealthy number of caffeine fueled sleepless nights, we finally submitted our work, incredibly proud of all we had pulled off. When our first round of reviews came in however, I was absolutely gutted by the amount of additional work we would have to do to see this publication through.
Around this time, I was also hit by tremendous personal loss, and grief felt like it would be the final straw that would break this camel’s back. We like to believe that we science in vacuum, but the reality is that we science despite the happenings of life, and we need to build a more sustainable culture that accommodates for it. While my instinct was to go full steam ahead with addressing reviewer comments, I had to take a step back and readjust my pace. I found a wonderful therapist, and eventually took some time away from work. While stepping on the brakes seemed counterintuitive at the time, it gave me the clarity and energy I needed to push through the review process. It also readjusted the insurmountable hopelessness I had felt with that first editorial decision. I am now utterly grateful for how much the reviewer’s suggestions helped improve the paper we finally ended up publishing.
When I look at my publication today, I can’t help but be struck by how 5 years of blood, sweat and tears coalesce so tidily into 5 pages of a journal. I wonder how differently things might have turned out, had I not had two amazing lab mentors pushing me along. I hope you are as fortunate as I am, and I hope, like me, you have supportive people around you. If you find yourself without an encouraging voice however, I hope you hear me when I tell you that your place in science is not accidental, and that your iceberg is much closer to the surface that you realize.
ABOUT: Stories in Science is a special series on the Civic Science Times. The main aim is to document the first-hand accounts of the human stories behind the science being published by scientists around the world. Such stories are an important element behind the civic nature of science.
SUBMISSION: Click here to access the story guidelines and submission portal. Please note that not all stories are accepted for publication. After submission, we will let you know whether we have selected the story for the review process.
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
 Civic Science Observer1 week ago
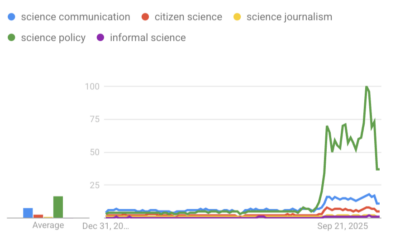
Civic Science Observer1 week ago‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress