Stories in Science Special Series
A non-linear path to the career I never knew I always wanted

By Valerie K. Haftel Ph.D | Associate Professor of Biology, Morehouse College, Atlanta, GA
[dropcap]I[/dropcap] was always fascinated by animals, and how they work in nature. I had a fondness for playing in streams with friends, catching frogs, collecting buckets of fish and trying to train them, playing with all manner of pets, and wanting to explore how they all work throughout my education. I was fascinated by physiology, even if I didn’t quite know what that meant. At the same time, I always had a feeling that I was different from everyone else. I was the youngest of three children of a Hispanic mom and Jewish dad living in a suburban, mostly white neighborhood. I had darker skin, dark curly hair, relatives that spoke a different language, relatives that practiced a different religion. These things, along with being quite introverted set me apart. No, this is not a story about a serial killer in the making. It’s a story about what good role models can do for a shy child who loved biology.
As a kid, I loved to learn, and compete with classmates to see who could finish assignments better and faster. I had a few really good teachers and role models in high school who inspired me to learn more, push harder and ask questions. They gave us things to do, brought in the newest information on science, and didn’t just expect us to memorize facts. My teachers were kind and gifted women scientists who worked hard to set us up for success. In fact, Doc Ellis (AP Biology teacher) working on her PhD while teaching and raising a family showed me that I could do all that and be an excellent, caring teacher and scientist. I held her up as a source of inspiration, and still do!
Strangely enough, the idea of becoming a scientist like Doc Ellis wasn’t on my radar. I wanted to be a “real” doctor! Apart from my High School role models, I didn’t see a lot of women scientists who also loved mentoring and education. I worked as a teaching assistant and tutor during undergrad, and continued as I acquired a MS in Physiology. After examining what it was that I was doing with my time, I finally realized that it wasn’t through medicine that I wanted to help others, but through education. In one of my graduate courses I learned about synaptic plasticity and was fascinated that a cellular change at a single synapse could be the answer to how to learn and remember! I enrolled in a PhD program to figure out how people learn and to gain experience to inform my future in education. Of course I found myself in a neurophysiology lab doing, you guessed it: spinal cord physiology (truly a good model of synaptic plasticity)!
The young, sharp minds in my lab and my courses teach me more every day about why it is so important to be here and contribute all the energy I can to ensure their success. Without these young men in our scientific work force, we would lose creative genius that would contribute to breakthroughs in science and technology in ways we may not be able to imagine right now.
I enjoyed the research and loved physiology, but struggled with my next steps, not having many role models to ask about transitioning into a career in science mentoring and teaching during my PhD program. Eventually, I had the difficult discussion about my future with my PI, and disclosed that a full time research-only career wasn’t for me. I wanted to incorporate research, but was drawn to teaching and mentoring science students to guide them to the right path and experiences for them to achieve their goals. In that difficult discussion, I planted a seed in his mind that enabled him to introduce me to a great opportunity that changed my life: the new NIH-funded FIRST Postdoctoral Fellowship program at Emory University. It wasn’t only the curriculum of the program but the links to dedicated people and historic places that made a difference in my career. The program focuses on training in innovative teaching techniques, while carrying out biomedical research, with an emphasis on working in Minority-serving institutions in Atlanta as role models of success in science/education. Mentors and opportunities in teaching at Spelman College, one of the most prestigious Historically Black Colleges in the world, highlighted the need for mentors to do better by the African American community in encouraging young minds to thrive in science and technology.
I felt I could contribute because in some ways I identified with feelings of being set apart as a child for my heritage, and I wanted to prevent others who may feel the same way from becoming discouraged. I wanted to merge my love of science and the support I had growing up with the desire to teach and mentor a group of students who don’t always have the support they need. I was fortunate to obtain job at Spelman’s sister institution, the equally prestigious Morehouse College. I felt this was the best fit for me to continue my work in research, teaching, mentoring, and really caring for my students where I could make a difference. This was also a time for me to become independent as a researcher. For this, I drew inspiration from my research experience in neural regeneration and plasticity, and the prevalence of diabetes and its secondary complications in my family, the Hispanic, and African American communities. These connections are essential to highlight, as the inspiration for my work comes from diverse sources, yet ties them together in fundamental ways. We need to support the communities that are affected by these issues to supply the researchers and health care providers and educators surrounding these problems, and to come together to create the solutions.
The rewards of my career in science teaching and mentoring have been immeasurable. Each one of my students who I mentor in my research lab has become part of my family. The young, sharp minds in my lab and my courses teach me more every day about why it is so important to be here and contribute all the energy I can to ensure their success. Without these young men in our scientific work force, we would lose creative genius that would contribute to breakthroughs in science and technology in ways we may not be able to imagine right now. Those few who may fall through the cracks teach me that there is more work to be done and not enough resources to support them. And so we work harder.
Photo by Wellcome Trust |No changes were made to the original image | Some rights reserved
The CS Media Lab is a Boston-anchored civic science news collective with local, national and global coverage on TV, digital print, and radio through CivicSciTV, CivicSciTimes, and CivicSciRadio. Programs include Questions of the Day, Changemakers, QuickTake, Consider This Next, Stories in Science, Sai Resident Collective and more.

-
Audio Studio1 month ago
“Reading it opened up a whole new world.” Kim Steele on building her company ‘Documentaries Don’t Work’
-
 Civic Science Observer1 week ago
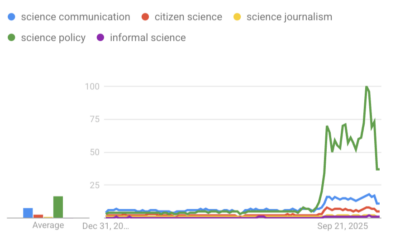
Civic Science Observer1 week ago‘Science policy’ Google searches spiked in 2025. What does that mean?
-
Civic Science Observer1 month ago
Our developing civic science photojournalism experiment: Photos from 2025
-
Civic Science Observer1 month ago
Together again: Day 1 of the 2025 ASTC conference in black and white
Contact
Menu
Designed with WordPress